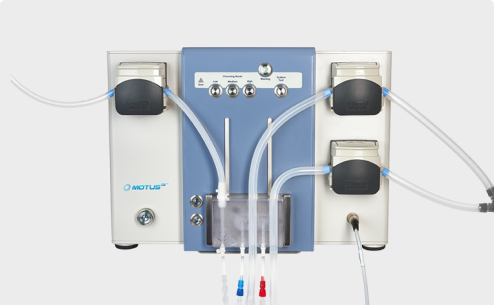
FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...
FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...

FDA has cleared a Motus GI Holdings 510(k) for the Pure-Vu EVS System for colonoscopies. The device is intended to improve speed of set-up...